


Interesting links:
Infrared through the ages
Infrared radiation was discovered by Isaac Newton when he separated the electromagnetic energy from sunlight by passing white light through a glass prism that broke up the beam into colors of the rainbow. In the 1800's, William Herschel discovered energy beyond the visible. In the 1900's, Planck, Stefan, Boltzmann and Wien further defined the activity of the electromagnetic spectrum and developed equations to identify IR energy. This research makes it possible to define IR energy using the basic blackbody (a perfect emitter) which shows that an object with a temperature greater than -273 deg C emits radiant energy in an amount proportional to the fourth power of their temperature.
Today Infrared Heating is used in the industry for a variety of applications as well as for comfort heating.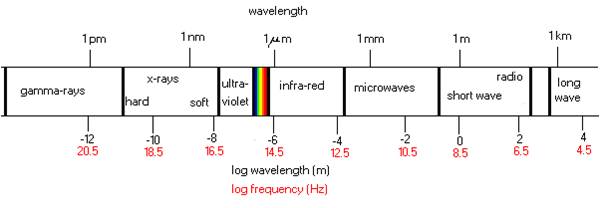
How does it work?
Infrared energy is heat that can be applied to many different things for manufacturing, finishing, drying and heat processing. To find out what infrared energy can do, and where it can and cannot be used to its full advantage, here is a short guide.
Essentially, infrared is an electromagnetic phenomenon, which is measured in wavelengths (µm = microns). Electromagnetic energy particles attack the surface of materials to be processed after which conduction takes over. To use infrared energy successfully, we have to understand this reaction. Materials can act as a good heat conductor (example: gold, copper). In many cases, however, the conductivity is less than desired resulting in absorbing and retarding the penetration of heat. Some of the materials may even work as an insulator. (example: foam, ceramic)
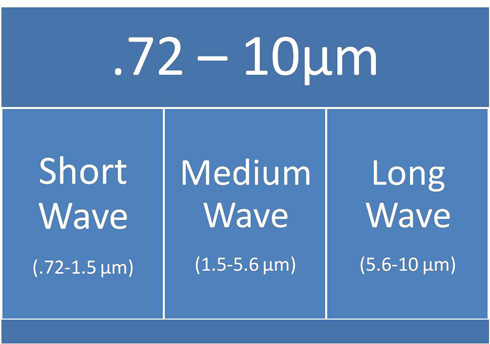
The infrared heater selection is essential to success. The infrared spectrum can be divided into roughly three types of density: short wave, medium wave and long wave. It is really important to understand that infrared energy is a surface phenomenon. For example, processing foam is poor radiant energy conductor so it should be radiated from both sides. Also, if the surface is shiny (example: aluminum), the surface may reflect the infrared waves and bounce back and forth which will result in a delayed heating action. Objects with surfaces that readily absorb energy usually give satisfactory results.
In industrial heating, the main thing to know is what is being processed and at what speed it is being processed. Basically, any product has its own inherent reaction to infrared. This is called "heat absorption factor". Each type of material can be categorized in a certain wavelength. Naturally, that can be translated into degrees of temperature. As a result, we are able to obtain the fastest possible reaction from materials if exposed to an infrared radiation peak corresponding to its absorption factor.
In all cases, infrared is a hotter energy source than convection heat. Infrared heat is always applied directly to materials. The exposure to this direct heat source has to be timed in order to not overheat and destroy the material.
To remember: for each material the right wavelength (= IR heater) must be chosen.
Infrared wavelength
The infrared wavelength spectrum is divided into three groups:

This is an infrared image of a Jet Propulsion Lab engineer holding a burning match. The image is color-coded to show differences in temperature: note the white and deep red in the flame and the engineer's palm (where his warm blood vessels are close to the surface of the skin) and the blue of his cool glasses. This picture demonstrates that infrared images predominantly show heat energy and its distribution.
|