


Heat is always transferred from the medium with higher temperature to the medium with lower temperature.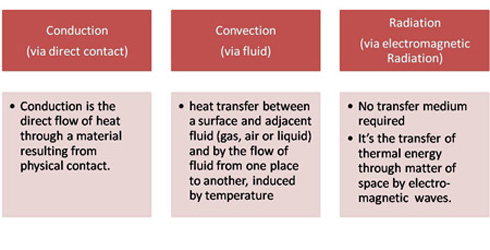
All three heat transfer methods have their application fields. For example a mold is best heated by an inserted heater (conduction), while glass can be bent using infrared heaters. Oftentimes a combination of incident heat is used (like radiation combined with convection)
Heating by conduction
The smoother the surface, the better is the heat transfer. Air gaps between the heater and the surface to be heated can reduce heater performance.
Heating by convection
Convection in the most general terms refers to the movement of molecules within fluids (i.e. gas, air or liquids). This movement can be natural or forced. In industrial applications, it is mostly better to force this movement. Air heaters require a constant airflow passing over the heating element; otherwise the heating element will burn out (especially if there is no high temperature sensor and flow meter installed!). This minimum airflow depends on the heater type and wattage. In liquids, this movement can be done by installing some kind of stirring mechanism into liquids so the immersion heater can transfer the heat properly.
Heating by radiation (Thermal Radiation)
The electromagnetic spectrum covers an enormous range in wavelength, from very short waves to very long ones.
The only region of the electromagnetic spectrum to which our eye is sensitive is the "visible" range identified by the rainbow colors.
In industrial heating, the radiation normally used is in the Ultraviolet-, Infrared-, Microwave- or short Radio wavelength.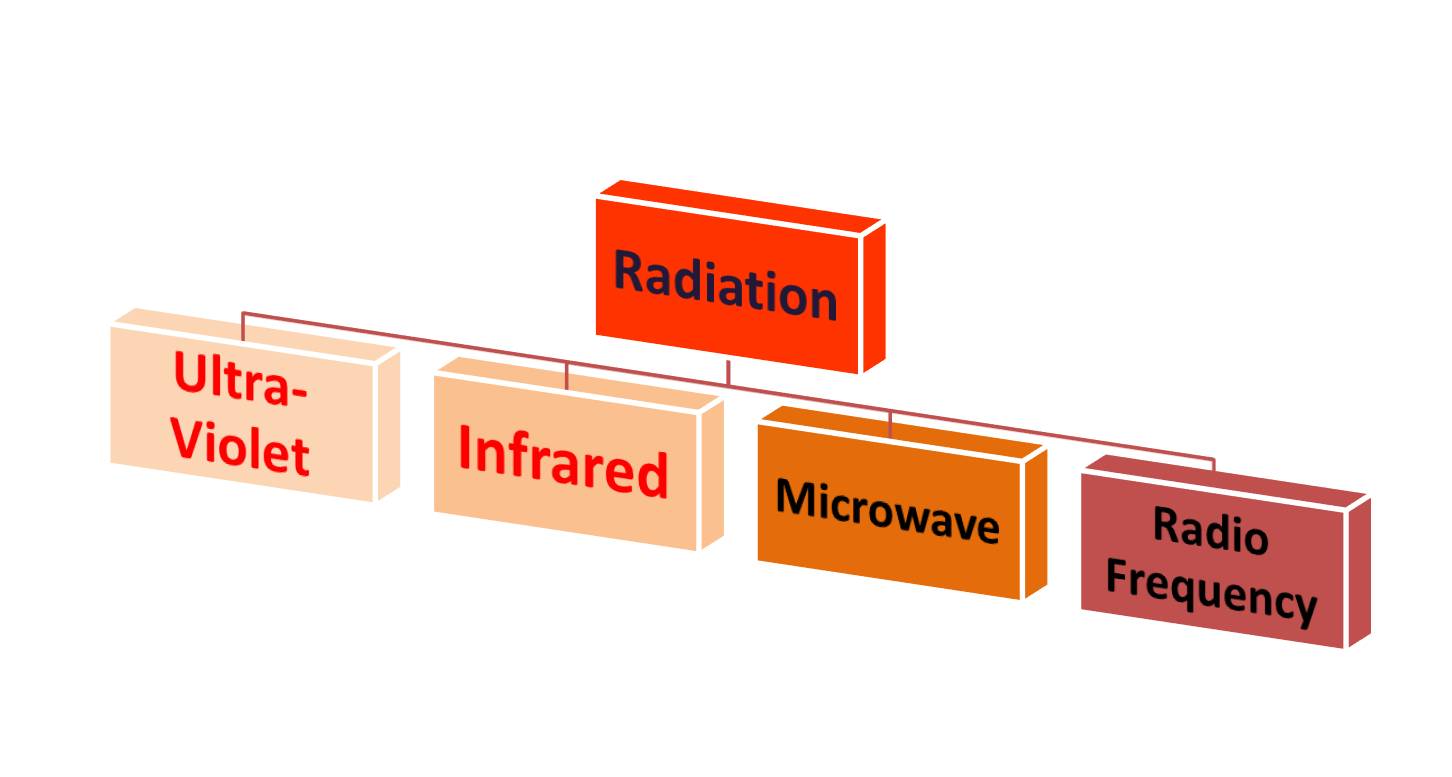
Infrared is only one in four ways to transfer heat via radiation
The sun is the biggest, but not the only object that provides radiant energy; any object whose temperature is greater than 0 K will emit some radiant energy. The human body has a temperature of about 310 K and radiates primarily in the far infrared.
The challenge to scientists was to show how this radiant energy is related to the temperature of the object.
If an object is placed in a container whose walls are at a uniform temperature, we expect the object to come into thermal equilibrium with the walls of the enclosure and the object should emit radiant energy just like the walls of the container. Such an object absorbs and radiates the same amount of energy. Now a blackened surface absorbs all radiation incident upon it and it must radiate in the same manner if it is in thermal equilibrium. Equilibrium thermal radiation is therefore called black body radiation.
The first relation between temperature and radiant energy was deduced by J. Stefan in 1884 and theoretically explained by Boltzmann about the same time. It states:![]()
where the total energy is per unit area per second emitted by the back body, T is its absolute (thermodynamic) temperature and ![]() is the Stefan-Boltzmann constant.
is the Stefan-Boltzmann constant.
The great question at the turn of the century was to explain the way this total radiant energy emitted by a black body was spread out into the various frequencies or wavelengths of the radiation. Maxwell's "classical" theory of electromagnetic oscillators failed to explain the observed brightness distribution. It was left to Max Planck to solve the dilemma by showing that the energy of the oscillators must be quantized, i.e. the energies can not take any value but must change in steps, the size of each step, or quantum, is proportional to the frequency of the oscillator and equal to hv, where h is the Planck constant. With this assumption, Planck derived the brightness distribution of a black body and showed that it is defined by its temperature. Once the temperature of a black body is specified, the Planck law can be used to calculate the intensity of the light emitted by the body as a function of wavelength. Conversely, if the brightness distribution of a radiating body is measured, then, by fitting a Planck curve to it, its temperature can be determined.
The curves illustrated below show that the hotter the body is, the brighter it is at shorter wavelengths. The surface temperature of the sun is 6000 K, and Planck curve peaks in the visible wavelength range. For bodies cooler than the sun, the peak of the Planck curve shifts to longer wavelengths, until a temperature is reached such that very little radiant energy is emitted in the visible range.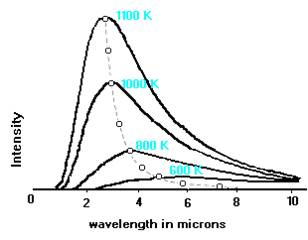
This figure (adapted from Adkins' "Thermal Physics") shows several Planck curves for black bodies. The Intensity is in units of energy per unit area per unit solid angle per unit time per unit wavelength interval. The broken line illustrates the variation with wavelength and temperature of the peaks of the curves.
This is a representation of Wien's law, which states:![]() (max) ~ 0.29/T,
(max) ~ 0.29/T,
where ![]() (max) is the wavelength of maximum brightness in cm and T is the absolute temperature of the black body.
(max) is the wavelength of maximum brightness in cm and T is the absolute temperature of the black body.
The main infrared laws: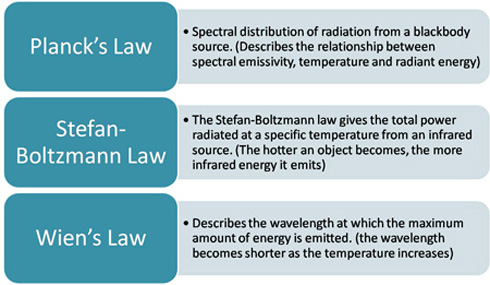
The most important thing to know:
All radiation is either reflected, absorbed or transmitted.
|